In the atom the basic view of the atom is Proton and neutron tightly packed in very small volume inside nuclues and the electrons rotating like planets around the nucleus. As discussed at the microscopic world the wave nature of matter become important. So No! No! we can't see the atom as our planetary system.
 |
| NO NO This is not the Right model of atom |
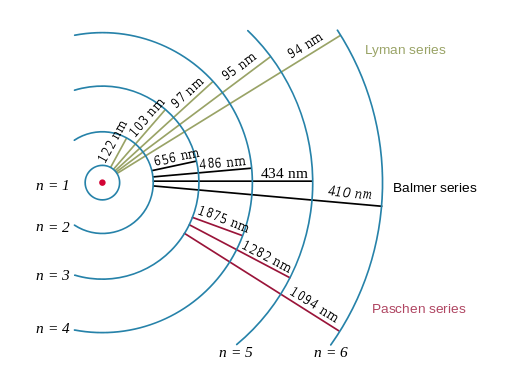 |
| The electron transitions in hydrogen atom |
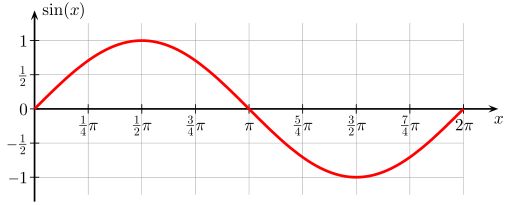
See other sinsuidal wave having two complete wavelength and completing the 0 to 4 pie.
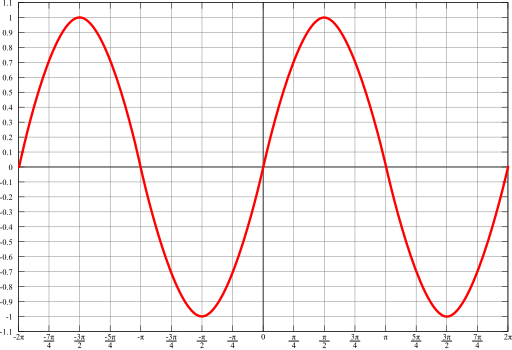
But Electron have to revolve in a 2 Pie space or 360 degree and to complete its two wave length in same space.
 |
| Circular standing wave |
For Science ebook in hindi please visit www.upstudent.blogspot.com
No comments:
Post a Comment